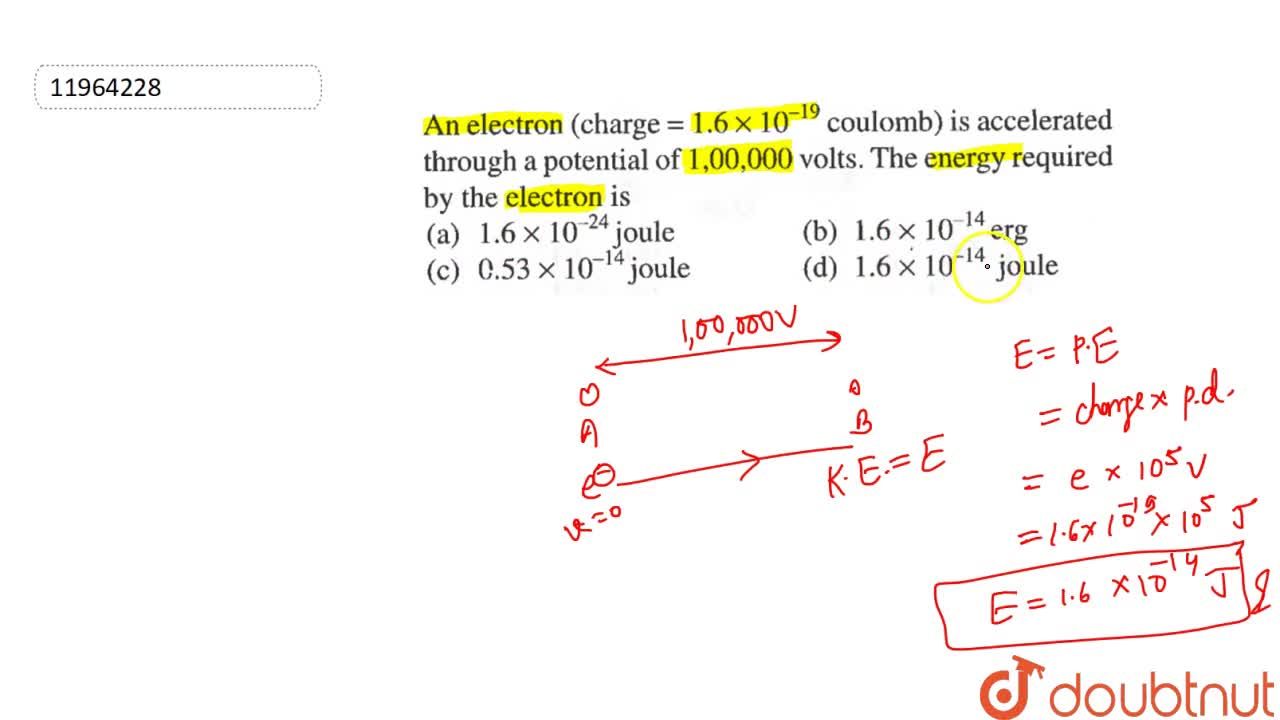
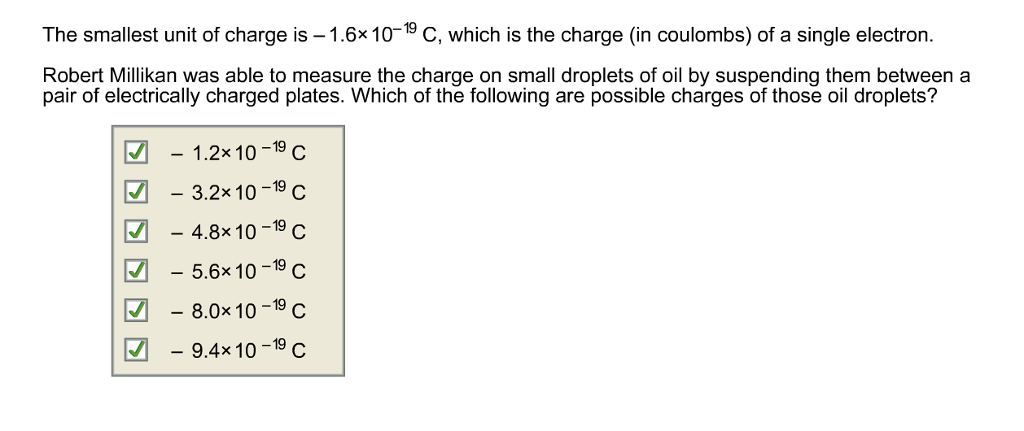
Charge on an electron is 1.6 × 10^-19 coulomb. Number of electrons passing through the wire per second on flowing of 1 ampere current through the wire will be

If the charge on an electron be 1.6×10-19 C, find the approximate number of electron in 1C ? - Brainly.in
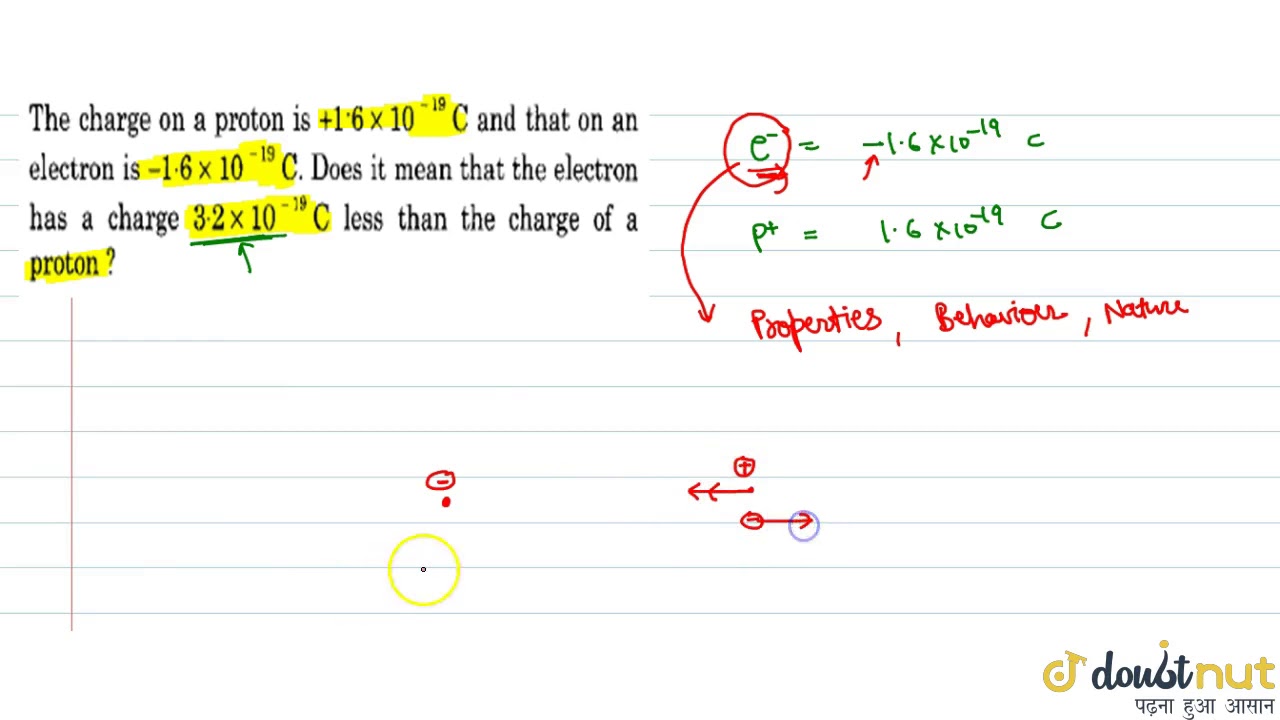
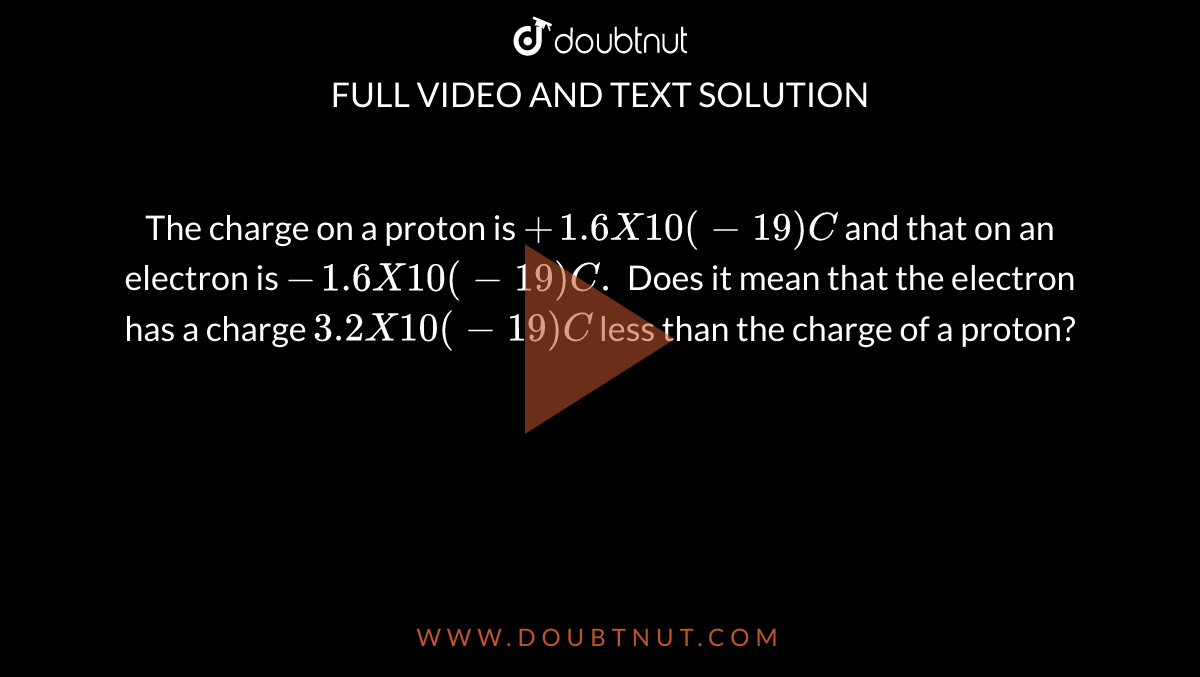
The charge on a proton is `+1.6 X 10(-19) C` and that on an . electron is `-1.6 X 10 (-19)C.` Do... - YouTube
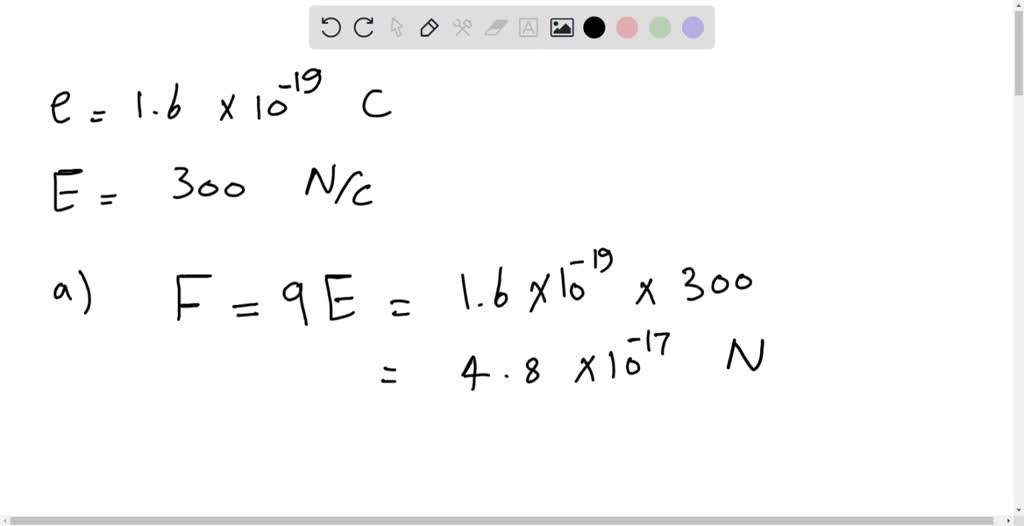
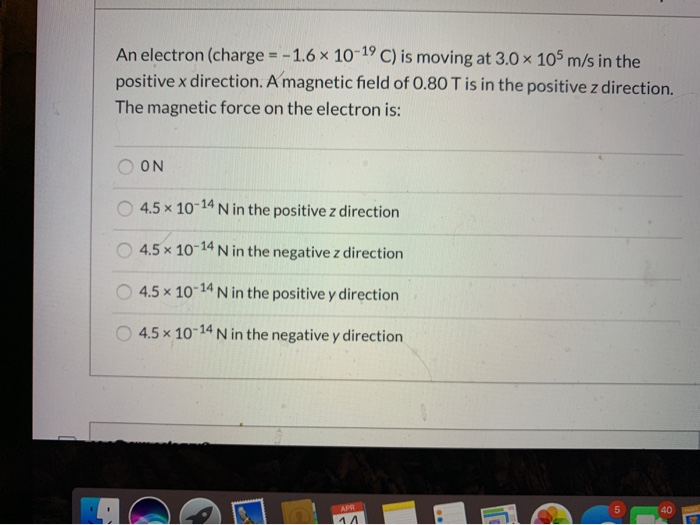
SOLVED: An electron with a charge value of 1.6 x10^-19 C is moving in the presence of an electric field of 300 N/C. a) What electric force does the electron experience? b)

Electrostatics Electro- Electricity/charged particles Static-stationary/ not moving Electrostatics - the study of stationary charges Do NOW: What do you. - ppt download
The charge on an electron is 1.6×10^-19C. How can I find the number of electron that flows per second to constitute a current of 2A? - Quora

Electricity Sections 12.1 and Reminders In-class Quiz #5 Tuesday, November 4 LAB this week B3-CLE: Coulomb's Law of Electrostatics Mallard-based. - ppt download

Charge on an electron is 1.6 × 10^-19 coulomb. Number of electrons passing through the wire per second on flowing of 1 ampere current through the wire will be

Charge on an electron is 1.6 × 10^-19 coulomb. Number of electrons passing through the wire per second on flowing of 1 ampere current through the wire will be
What does [math]1.6\times 10^{-19}[/math] coulomb mean? Yes, it's the charge of an electron, but what does that really mean? - Quora

the charge on an electron is 1.6 into 10 to the power minus 19 coulomb find the number of electrons that - Brainly.in

If the charge of an electron is 1.6 x 10-19 coulomb, how many electrons should pass through a conductor in 1 - Brainly.in

The charge possessed by an electron is 1.6 X 10-19 coulombs. Find the number of electrons that will flow per - Brainly.in

THE CHARGE ON AN ELECTRON IS 1 6 *10^-19C FIND THE NUMBER OF ELECTRONS THAT WILL FLOW PER SECOND - Science - Electricity - 13646369 | Meritnation.com

The charge of an electron is `1.6 xx 10^(-19)C` what will be the value of charge on `Na^(+)` ion. - YouTube