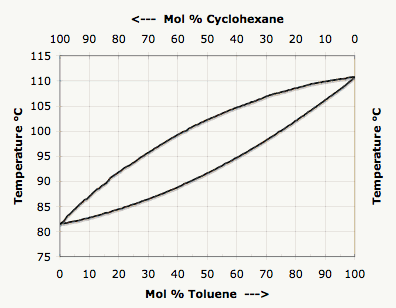
SOLVED: Chapter 5 Distillation FIG. 5.3 Boiling point - composition curves for a mixture of cyclohexane and toluene 110 110 Vapor 0100 L 100 Liquid 100% Cycl 0"0 Tol. Cycl 0% Tol. 100% Mole percent cyclohexane Mole percent toluene -
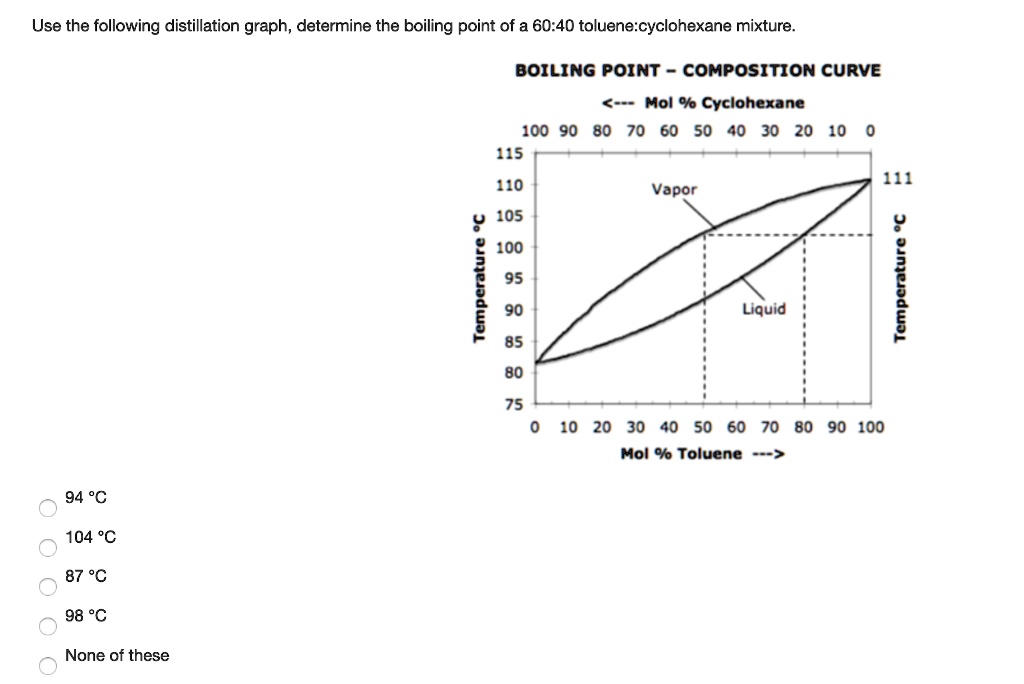
SOLVED: Use the following distillation graph, determine the boiling point of a 60.40 toluene:cyclohexane mixture. BOILING POINT COMPOSITION curve 0 Mol % Cyclohexane 10090 80 70 60 50 40 30 20 10

The melting points from benzene to cyclohexane: a prime example of dispersion forces in action? | Henry Rzepa's Blog

Assuming that water vapour is an ideal gas, the internal energy change (Δ U) when 1 mol of water is vapourised at 1 bar pressure and 100 ^0 C will be: [Given
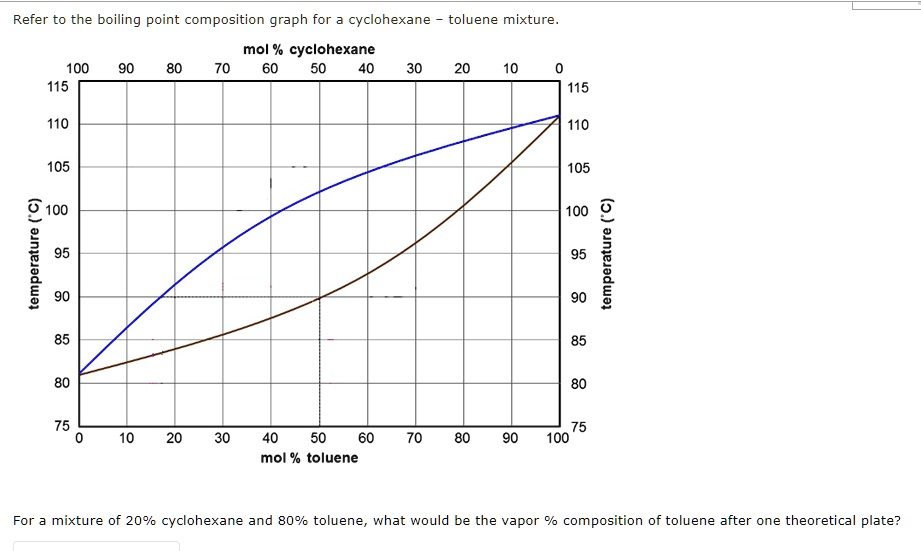
SOLVED: Refer to the boiling point composition graph for cyclohexane toluene mixture mol % cyclohexane 100 115 115 110 110 105 105 2 100 L 100 2 | 100 mol % toluene

organic chemistry - Boiling point comparison between cyclohexane derivatives - Chemistry Stack Exchange

Assuming that water vapour is an ideal gas, the internal energy change (Δ U) when 1 mol of water is vapourised at 1 bar pressure and 100 ^0 C will be: [Given