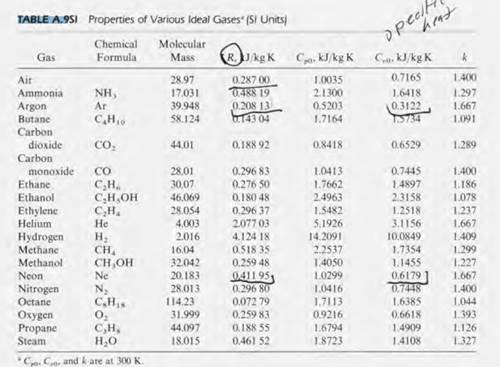
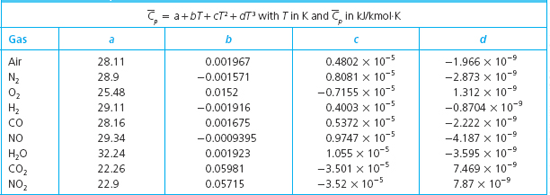
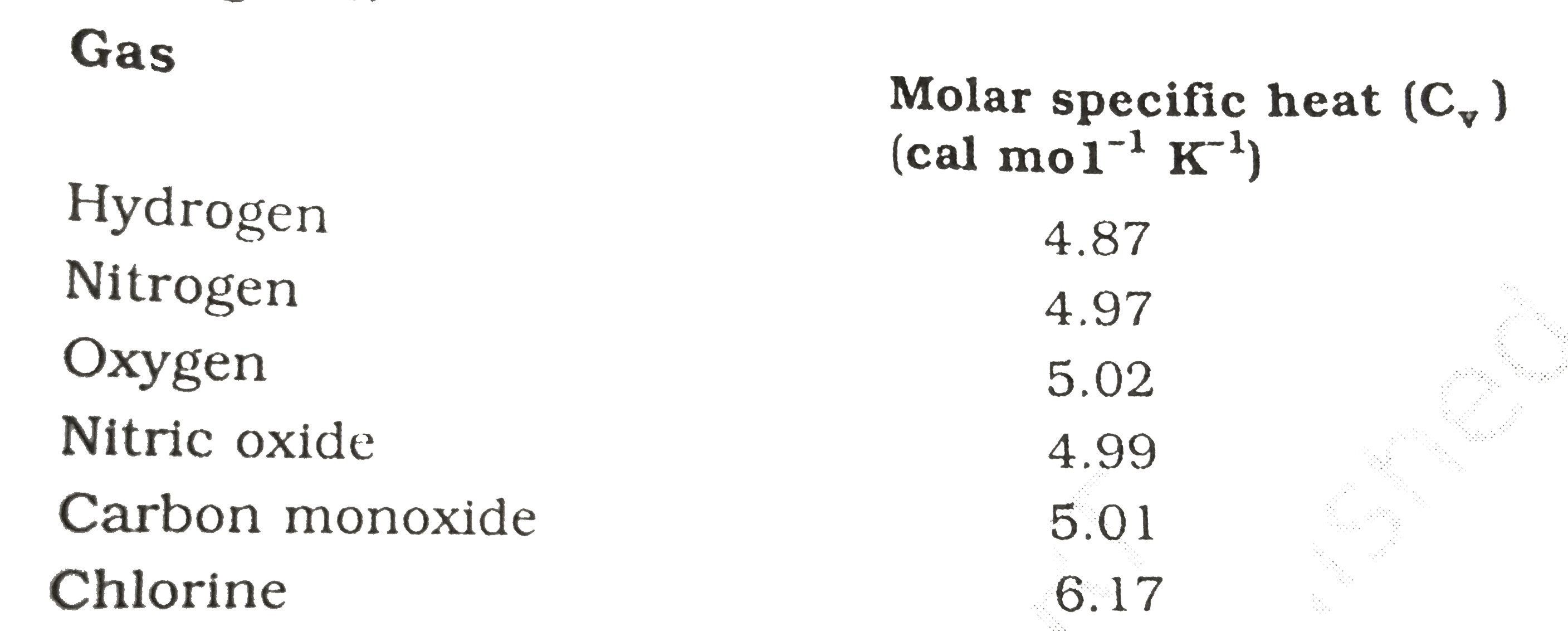The Molar heat capacities of nitrogen at constant pressure and constant volume are 29.11 kJ/k mole K and 20.81 kJ/kmole k, respectively. When 5 gram of nitrogen is heated from 290 to

SOLVED: The constant pressure molar heat capacity of nitrogen gas, N2, is 29.125 J K–1 mol–1 at 298.15 K. Calculate the change in the internal energy when 2.00 mol of nitrogen gas

This week in the physics course Lectures will cover Chapter 16 (Temperature and Heat) and start Chapter 17 (Thermal Behaviour of Matter) Tutorial class. - ppt download

Table 1 from Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa | Semantic Scholar

Density and specific heat of nitrogen as function of temperature. Data... | Download Scientific Diagram
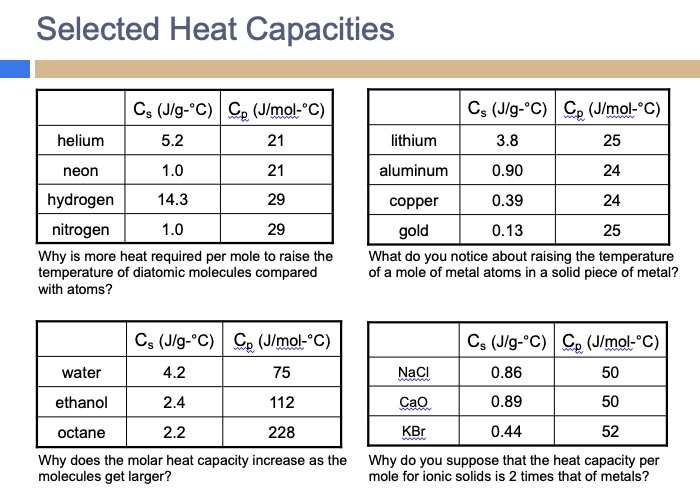
SOLVED: Selected Heat Capacities Cs (Jlg-"C) Cp (Jlmol:"C) helium 5.2 Cs (Jlg-"C) Ce (Jlmol-"C) lithium 3.8 25 neon 1.0 aluminum 0.90 24 hydrogen 14.3 29 29 copper 0.39 24 nitrogen 1.0 Why

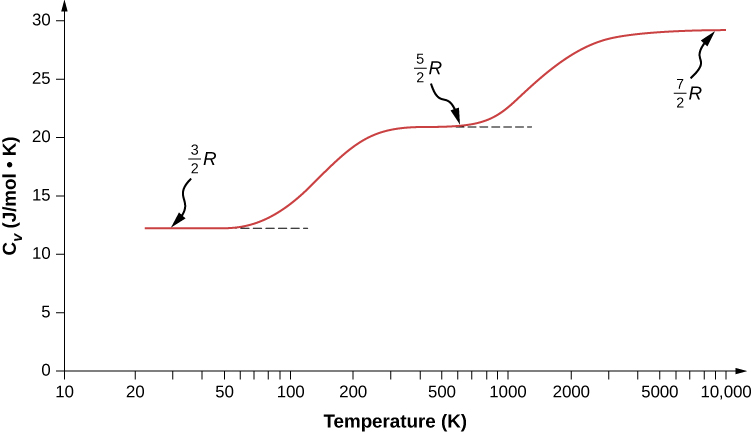
The molar heat capacity at constant pressure of nitrogen gas at `STP` is nearly `3.5 R`.Now when the - YouTube

Calculate the ratio of specific heats for nitrogen. Given that the specific heat of nitrogen at - YouTube

The temperature of 3 kg of nitrogen is raised from 10 ^o C to 100 ^o C. Compute the difference in work done if heating were at constant pressure and at constant